How to Teach Elements, Compounds and Mixtures Visually
- Androy

- Sep 24, 2022
- 7 min read
A Step by Step lesson guide with examples
In this post, I discuss how I use ideas from Johnstone’s chemistry triangle to teach students all about elements compounds, and mixtures in a meaningful way, setting the foundation for other topics such as writing and understanding chemical formulae and balancing equations. I use particle models in most of my explanations, as a visual tool that helps reduce cognitive load and leads to conceptual understanding.
You may choose to sequence your lesson differently than I do here as this is highly dependent on the student's prior knowledge, however, the way that I have decided to sequence this topic is based on a year-long action research project I conducted in 2017 which had favourable results. You may want to take notes.
A useful resource for reviewing Elements, Compounds and Mixtures
Using Johnstone’s Chemistry triangle to teach Elements, Compounds, and Mixtures
Alex Johnstone (1982) suggests that chemistry knowledge is understood at three levels of representation:
The macroscopic level: this is the observable chemical phenomenon that students encounter every day. This level involves what can be observed in the laboratory such as bubbles forming during a chemical reaction, or a colour change.
The symbolic level: is used to communicate the phenomena at the macroscopic level, and this includes graphs, chemical equations, chemical formulae, and reaction mechanisms
The submicroscopic level: is based on the particulate theory of matter and makes use of models to explain the macroscopic phenomena in terms of the movement or arrangement of particles such as electrons, molecules, and atoms that are too small to be observed.
Research shows that for deeper understanding and meaningful learning to occur students need to understand chemistry at all three levels of representation and should therefore be taught using all three levels (Treagust and Harrison, 1999; Johnstone, 1982).
So what would Johnstone’s triangle look like for elements compounds and mixtures?
Start with Pure Substances and Mixtures
Matter can be classified as pure substances and mixtures, which can be further classified as shown below.
In chemistry, materials are either pure samples of a single substance or a mixture of substances. This idea is easy enough for students to contend with. When a material is a mixture it can, in principle be separated into its component parts using a number of different separation techniques. If a substance is pure then it only has one component and thus there is no need for separation.
When introducing these ideas, not only is it important to define what is meant by a pure substance and a mixture, but it is also beneficial to indicate the properties of each;
Pure substances:
Have fixed and constant compositions
Have fixed and constant properties e.g. melting point, boiling point
The component parts cannot be separated by any physical process
Mixture
Have variable composition i.e. it is not uniformed
The properties are variable (because its component parts keep their individual properties)
Its component parts can be separated by physical means.
Students need to understand what these properties mean. To do these three activities which demonstrate these properties at the macroscopic level of representation can be used.
Activity 1: Mixtures vs Pure substances sorting activity
Students are placed in small groups and each group is given samples of mixtures and pure substances from the laboratory, placed in boiling tubes with rubber bungs attached. Within their groups, students are asked to sort the samples into two groups, mixtures, and pure substances, and to provide reasons for their answers. This activity opens up the discussion regarding the properties of mixtures and pure substances.
Examples of substances given to each group of students:
Sand mixed with salt
Iron filings with sulfur powder
Iron filing with copper
Sugar mixed with salt
Iron
Copper
Zinc granules
Salt ( sodium chloride)
Copper sulfate crystals
Glucose powder
For this introductory activity, I start off with only heterogeneous mixtures as homogeneous adds an additional challenge and will be tackled later.
Activity 2: Crude separation of the mixtures
What does it mean to be separated by physical means? This activity demonstrates what we mean by separating a mixture by physical means.
At this point, older students usually have some general ideas of the separation techniques. You can use this activity to review the concepts.
I ask students to explain (in their groups) how they can separate the components of the mixtures they have sorted out from activity 1.
A simple demonstration with iron filings and sulfur using magnets can be done. Compare this with one of the pure samples.
Activity 3: Experimentally differentiating between mixtures and pure substances
If you have the time available, after conducting the first two activities, you can demonstrate that pure substances have fixed and constant properties whereas impure substances (mixtures) have variable properties by performing a simple laboratory activity comparing the boiling point of pure water to that of a solution of sodium chloride.
This practical activity shows that pure water has a sharply defined boiling point, however, a mixture of sodium chloride and water boils over a range of temperatures.
This activity is more useful if you have already covered changing states and interpreting heating and cooling curves.
Teaching about elements and compounds
The difference between elements and compounds is a fundamental idea in chemistry that all students should appreciate. However, unlike pure substances and mixtures, there is no immediate way to distinguish between elements and compounds. They are both pure substances and this is where a bit of confusion (and misconceptions) can ensue.
I have found that once students have learned about atoms it is easier to teach and define an element as a substance that only has one type of atom present and a compound as a substance made up of different atoms that are chemically combined to form molecules.
I prefer the use of these definitions because unfortunately "element" can be defined based on the macroscopic level i.e substances and their reactions which is different from the definition of element at the submicroscopic level referring to the atom. I minimize cognitive overload by presenting elements and atoms as two separate definitions.
Connecting the Macroscopic with the submicroscopic using particle diagrams
We have now established what pure substances are and what mixtures are, now we focus solely on pure substances.
Elements
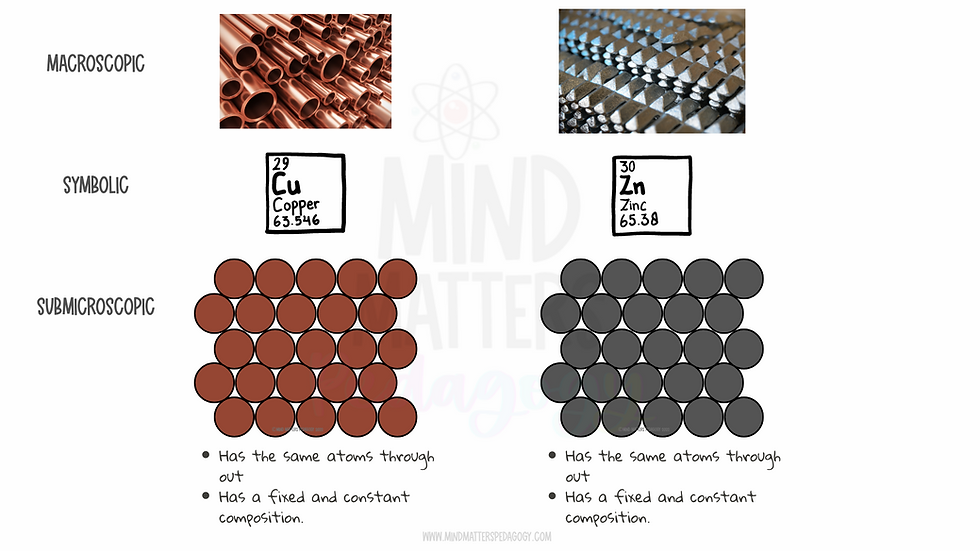
To explain elements as pure substances students are given samples of pure substances as described earlier. For example, I would have students observe zinc and copper and explain that these are pure because they contain only zinc atoms or only copper atoms. Then this is shown using particle diagrams. I use similar colours to represent atoms with identical atomic cores.
By presenting these ideas visually students begin to see (and process) that :
Elements are different from each other because they have different atoms (represented using different colours or sizes)
Elements are pure because they contain only one type of atom.
It is also important to show these “elements” in different states. E.g as molecules, as single atoms (the noble gases exist as single atoms), tightly packed solids representing metals, etc.
Compounds:
This activity is repeated for compounds. Two (or three) examples of compounds are selected from the introductory activity (It is important here to use the same examples as before so that students are able to make connections with what they previously observed and discussed).
For example, pure water contains only water molecules. I'll explain this by presenting a particle model containing water molecules again with each atom represented by different colours. I explain that it is a pure substance because it contains ONLY water molecules and nothing else.
The submicroscopic representation is useful here because:
Students can see that the atoms are chemically combined or "stuck together" and each "molecule" or unit should be considered as one single component.
N.B. It is useful if students know the difference between chemical and physical changes for the next section.
How are compounds pure substances??
The more astute student will then ask, how are compounds considered pure if they are made up of different atoms? Should these not be considered mixtures? If your students do not bring this up. You definitely should! Allow them time to think about this idea and propose a reason before going into any further explanation.
First students should be allowed to review the characteristics of pure substances and mixtures. Make sure to highlight the fact that pure substances have fixed and constant compositions and mixtures do not.
Then demonstrate this on the submicroscopic level as I do in the diagram below:
These diagrams show two things:
These substances have a fixed composition. In the case of the solid explain this with the alternating colours. A repeating pattern such as found in a solid crystal lattice like sodium chloride.
The atoms although different are stuck together and cannot be pulled apart without lots and lots and lots of energy.
Compare this with a diagram of a mixture such as the one shown below:
Explain that:
The substance does not have a fixed composition
The atoms are not chemically bonded (or stuck together)
Once we have covered these pure substances then we can revisit mixtures in more detail.
Mixtures and Their Separation
Mixtures consist of two or more substances that are physically combined in variable proportions. Each component of the mixture retains its own individual properties and is not chemically bonded to any other component of the mixture.
Emphasis should be placed on the ideas in bold type of this definition. If you have been following this lesson structure, then by this point students should already understand what mixtures are and some of the properties of mixtures.
The difficulty introduced when teaching mixtures in more detail is homogeneous mixtures. This is the point where the distinction between a mixture and a pure substance becomes less obvious.
It is easier enough for students to see that sand in water is a mixture, however, a solution of sodium chloride or copper(II) sulfate is less obvious.
To really distinguish between a pure substance and a mixture (both homogeneous and heterogeneous) you need to connect the macroscopic with the submicroscopic and explain how such mixtures as those mentioned below can be explained at the particle level.
Activity 1: Show the composition of mixtures at the submicroscopic level
Use a particle diagram of sodium chloride, then of pure water then of a sodium chloride solution like the one shown below. Explain how for the pure sodium chloride and the water the substance has a uniform composition and is made up of only one component. Then when the two are mixed, even though we can't see it on the macroscopic level, the composition of the mixture is not uninformed as we would imagine. Sometimes I make a show of actually mixing some salt with water and stirring to form a solution, then bring my students' attention to the particle diagrams.
The diagram below shows how I represent a solution of sodium chloride at the submicroscopic level:
Activity 2: Demonstrate that mixtures can be separated by physical means: even though they appear to be homogeneous
Now students can perform some laboratory activities where they get to separate some homogeneous mixtures such as ink using chromatography, or some solutions using simple and/or fractional distillation etc.
Elements, Compounds and Mixtures Digital Learning Resource
Based on the ideas here I created a digital resource which appeals to visual as well as kinesthetic-tactile learners. With this resource, students get to visually manipulate particles to create elements, compounds and mixtures, and sort through and classify different substances. Each activity is classified based on Bloom's revised taxonomy to elicit higher-order thinking.
This is a great resource for getting students thinking about these ideas.

























Comments